On This Page:
The nucleus accumbens is a region in the brain’s basal forebrain that plays a critical role in the reward circuit. It is involved in pleasure, reinforcement learning, and the processing of motivation and reward. Dysregulation of this area is often implicated in addiction, depression, and other psychiatric disorders.
Location
The nucleus accumbens (NAcc) is located in the basal forebrain and is a major structure of the ventral striatum, an area of the brain associated with the limbic system, and regulates limbic functions of motivation, affect, and reward.
There is a NAcc in each cerebral hemisphere, on the left and the right sides, and it is situated between the caudate and the putamen.
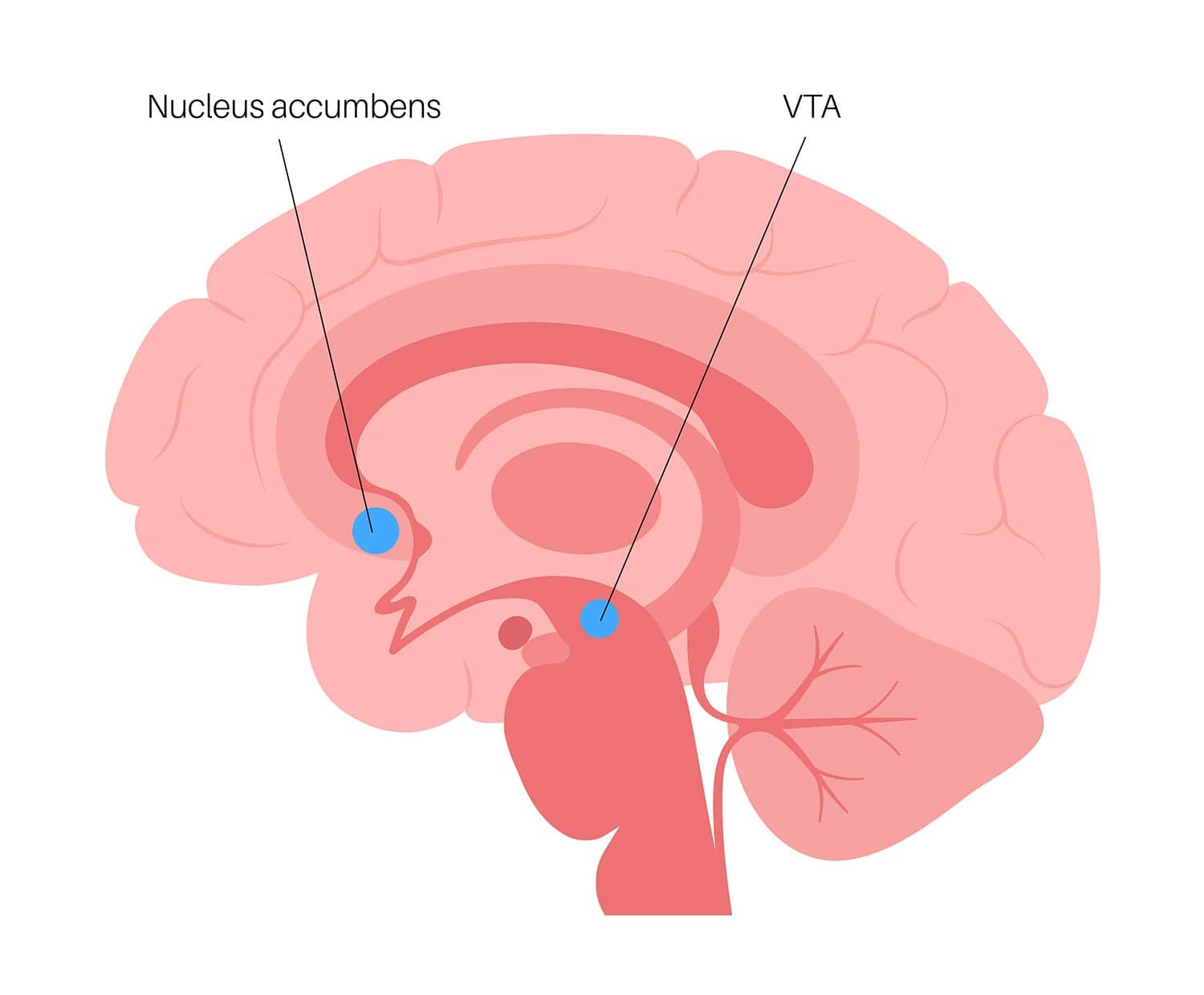
Historically, there has been disagreement over whether the NAcc belongs to the septal system (a region that has strong projections to emotion-generated areas) or the basal ganglia (a group of nuclei responsible primarily for motor control, executive functions, and behaviors).
After decades of research, the NAcc is suggested to be a specialized part of the striatal complex.
The NAcc is a round but dorsally flattened structure. Unique to the rest of the striatum, the NAcc can be divided into a central core which is surrounded by a shell.
The shell and the core have overlapping connections but may be responsible for making different contributions to the function of the NAcc.
In studies of rats, the shell appears to be more strongly associated with the limbic system, whereas the core is more strongly connected to the motor system.
The primary neurons of the NAcc are medium, spiny neurons, the development of which can be influenced by environmental factors. Dopamine is a major neurotransmitter of the NAcc.
Dopamine is a chemical messenger that plays an important role in motivation, arousal, reward, and motor control.
As a functionally central structure between the amygdala, basal ganglia, mesolimbic and dopaminergic regions, and the prefrontal cortex, it appears that the NAcc plays a modulative role in the flow of information from the amygdaloid complex to these regions.
Role of the nucleus accumbens in the ‘reward circuit ‘
The most widely recognized function of the NAcc is its role in the ‘ reward circuit ’ of the brain. The NAcc is a component of a major dopamine pathway of the brain called the mesolimbic pathway.
The mesolimbic dopamine pathway is stimulated during rewarding experiences. Essentially, when we do something which is rewarding, such as eating a favorite food or engaging in an enjoyable activity, the dopamine neurons in an area of the brain called the ventral tegmental area (VTA) are activated.
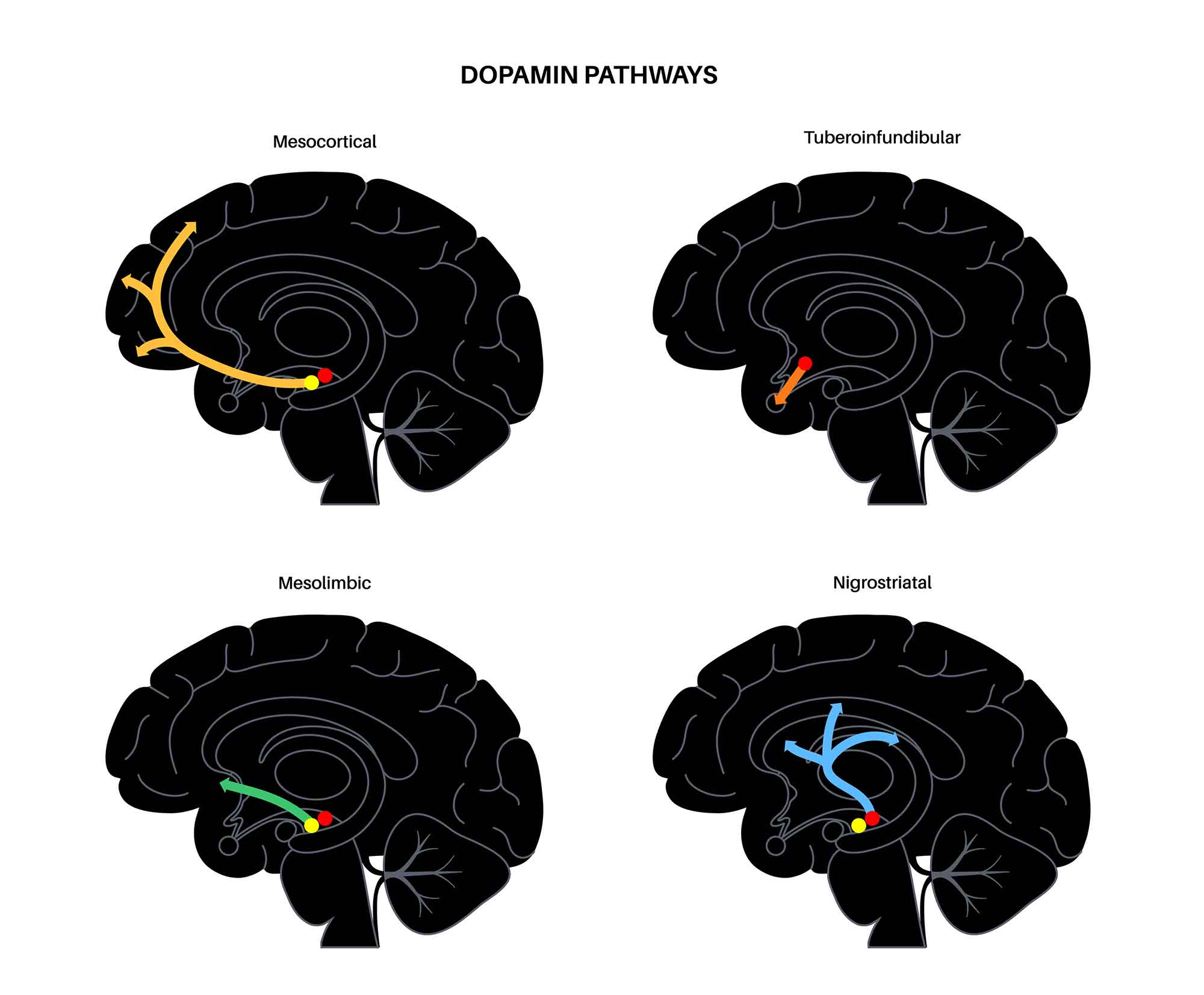
The VTA is a group of neurons located in the midbrain that play a key role in dopamine-related functions. These neurons in the VTA project to the NAcc, and when they are activated, it results in an increase in the dopamine levels in the NAcc.
The activation of the mesolimbic dopamine pathway can also tell us to repeat what just happened- the rewarding experience- to feel the rewarding sensation again.
For instance, you may receive a surge of dopamine to the NAcc due to eating a favorite food. Because of the pleasurable reward sensations that are experienced as a result of eating this food, you will be more likely to seek this food out again to achieve the feelings of reward.
The association between the rewarding experiences and dopamine levels in the NAcc initially caused neuroscientists to believe the main role of the NAcc is in mediating reward.
However, the role of NAcc is thought to be more complex than simply reward processing and its role is still not fully understood.
Since the initial associations between the NAcc and rewards have been found, it has since been discovered that dopamine levels in the NAcc increase in response to both rewarding and aversive stimuli.
A widely understood perspective now is that dopamine levels in the NAcc do not only rise during rewarding experiences but can rise anytime someone experiences something that can be deemed positive or negative.
Other Nucleus Accumbens function
The exact role of the NAcc in the processing of rewards is unclear and believed to be more complex than was originally thought. It is thought that the NAcc is likely involved in learning about rewards, as well as the stimuli that is associated with them.
The NAcc also seems to be important in stimulating reward-seeking behaviors and the selection of actions that are most likely to result in obtaining the rewards.
Alongside this, the NAcc is also believed to help suppress actions that are less likely to be useful in obtaining the reward.
The NAcc also appears to be important in processing aversive experiences and learning to move away from aversive stimuli. Therefore, the NAcc appears to be involved in motivational processes, whether rewarding or aversive.
Since the NAcc has a connection to the amygdala, a region of the limbic system associated with emotions, it is believed this region attributes feelings towards the rewarding or aversive experiences.
For instance, if eating a favorable food, the amygdala may attribute feelings of enjoyment and happiness to this stimulus. Assigning emotions to rewarding or aversive stimuli can further motivate us to either seek out the stimuli again or to avoid it.
The NAcc also has connections to the hippocampus, a region involved in memory. Through these connections, the hippocampus helps to attribute memories and learning to the rewarding or aversive experience.
For example, after eating a favorable food, the hippocampus can help to remember where the food was acquired from and remember the enjoyment of the food, motivating us to seek out this food again.
Essentially, the hippocampus, alongside the amygdala, work together to encourage the repetition of rewarding experiences.
Studies have implicated the NAcc in anticipation of incentives as well as in anticipation of rewards (Knutson et al., 2001). This shows that individuals can experience rewarding sensations before being exposed to the rewarding stimuli.
Further, the NAcc had shown greater activation for rewarding stimuli when the stimuli were unpredictable (Berns et al., 2001).
This illustrates that for pleasurable stimuli, the predictability of the stimuli can modulate the response of the NAcc, as subjective preference can be distinguishable from this response.
As well as incentive and reward, it has been demonstrated that the NAcc may play crucial roles in the following:
-
Locomotion
-
Learning
-
Avoidance
-
Impulsivity
-
Risk-taking behaviors
-
Sexual motivation
Due to input from the limbic system, as well as output similarity to the motor nuclei of the basal ganglia, the NAcc has been suggested to be the functional interface between the limbic and motor systems (Mogenson et al., 1980; Wise, 1982).
It has been found that dopamine in the NAcc of rats enhanced locomotor activity (Costall & Naylor, 1975), suggesting that a motor stimulant is partially mediated by dopamine receptors.
There also seems to be a divide of roles between the core and the shell of the NAcc. The shell is suspected of mediating the reinforcing properties of feeding behavior and rewarding substances and can influence the relapse of drugs.
Whereas the core seems to play a role in spatial learning, conditioned responses, responses to motivational stimuli, and impulsive choices.
Conditions associated with the nucleus accumbens
Substance use disorders
Typically, with repeated exposure to a reward, the rewarding feeling will wane. However, with drugs of abuse, the functioning of the reward system can become overwhelming since the effects of these drugs do not wane after repeated exposure.
This can be one of the reasons why people develop substance use disorders. Drugs of abuse tend to increase dopamine in the NAcc or change synaptic plasticity, whereas non-abused drugs generally do not affect dopamine in the NAcc or synaptic plasticity.
The mesolimbic dopamine pathway can become hypofunctional in the addicted brain, resulting in decreased interest in non-drug-related stimuli and increased dependence on the drug to experience rewarding feelings.
Mood disorders
As the NAcc is involved with cognitive and emotional functions, it may be involved in psychiatric conditions such as mood disorders. Patients with mood disorders have been found to have reduced NAcc activation (Heller et al., 2009).
Patients with mood disorders were also found to have reduced NAcc volume in comparison to those without mood disorders (Baumann et al., 1999). A study used deep brain stimulation (a therapy for severe depression that proves resistant to other treatment methods) on the NAcc of those with depression.
They found that after 12 months, 50% of the patients had significant reductions in their depressive symptoms (Bewernick et al., 2010). This implies that the NAcc may play a role in symptoms of depression.
Anxiety disorders
Due to connections to emotional regions of the brain, specifically the amygdala, the NAcc may be linked to anxiety conditions, which are also thought to be related to emotional regions.
It was found that fear memories and anxiety induced by stress appear to be influenced by mechanisms in the NAcc (Du et al., 2019).
Deep brain stimulation has been applied to the NAcc of patients with obsessive-compulsive disorder (OCD), an anxiety disorder, resulting in significant improvements of their symptoms and quality of life (Denys et al., 2010).
This further implies that anxiety disorders may be related to dysfunctions of the NAcc.
Parkinson’s Disease
Apathy, which is associated with a lack of interest and loss of initiative, is a complication of Parkinson’s Disease.
It has been found that these symptoms were associated with atrophy of the left NAcc in patients with this disease (Carriere et al., 2014), suggesting that the NAcc may have some involvement with this condition.
Alzheimer’s Disease
The NAcc is believed to be a brain region that is associated with cognitive impairments of Alzheimer’s Disease patients. Alterations of dopaminergic systems are frequently reported in those with Alzheimer’s, and these alterations are commonly linked with cognitive and non-cognitive symptoms.
It has been suggested that the VTA dopaminergic neuron degeneration results in lower dopamine outflow in the NAcc shell and hippocampus. This is said to be linked to impaired memory performance and dysfunctions of reward processing (Nobili et al., 2017).
Tourette Syndrome
The NAcc may also play a role in Tourette Syndrome. A study found that deep brain stimulation treatment has proved to be beneficial for reducing some of the syndrome’s motor manifestations, including alleviating tics and compulsions with the patient’s self-injurious behavior (Zabek et al., 2008).
Chronic pain
It has been suggested that those with chronic pain may have smaller NAcc volumes compared to those without chronic pain.
This has been suggested that the smaller NAcc may play a part in the risk of developing chronic pain (Makary et al., 2020).
References
Berns, G. S., McClure, S. M., Pagnoni, G., & Montague, P. R. (2001). Predictability modulates human brain response to reward. Journal of neuroscience, 21(8), 2793-2798.
Bewernick, B. H., Hurlemann, R., Matusch, A., Kayser, S., Grubert, C., Hadrysiewicz, B., Axmacher, N., Lemke, M., Cooper-Mahkorn, D., Cohen, M. X., Brockmann, H., Lenartz, D., Sturm, V. & Schlaepfer, T. E. (2010). Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biological psychiatry, 67(2), 110-116.
Carriere, N., Besson, P., Dujardin, K., Duhamel, A., Defebvre, L., Delmaire, C., & Devos, D. (2014). Apathy in Parkinson’s disease is associated with nucleus accumbens atrophy: a magnetic resonance imaging shape analysis. Movement disorders, 29(7), 897-903.
Denys, D., Mantione, M., Figee, M., Van Den Munckhof, P., Koerselman, F., Westenberg, H., Bosch, A. & Schuurman, R. (2010). Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Archives of general psychiatry, 67(10), 1061-1068.
Du, K., Lu, W., Sun, Y., Feng, J., & Wang, J. H. (2019). mRNA and miRNA profiles in the nucleus accumbens are related to fear memory and anxiety induced by physical or psychological stress. Journal of psychiatric research, 118, 44-65.
Heller, A. S., Johnstone, T., Shackman, A. J., Light, S. N., Peterson, M. J., Kolden, G. G., Kalin, N. H. & Davidson, R. J. (2009). Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proceedings of the National Academy of Sciences, 106(52), 22445-22450.
Knutson, B., Adams, C. M., Fong, G. W., & Hommer, D. (2001). Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience, 21(16), RC159-RC159.
Makary, M. M., Polosecki, P., Cecchi, G. A., DeAraujo, I. E., Barron, D. S., Constable, T. R., Whang, P. G., Thomas, D. A., Mowafi, H., Small, D. M. & Geha, P. (2020). Loss of nucleus accumbens low-frequency fluctuations is a signature of chronic pain. Proceedings of the National Academy of Sciences, 117(18), 10015-10023.
Mavridis, I. (2015). The role of the nucleus accumbens in psychiatric disorders. Psychiatrike= Psychiatriki, 25(4), 282-294.
Mogenson, G. J., Jones, D. L., & Yim, C. Y. (1980). From motivation to action: functional interface between the limbic system and the motor system. Progress in neurobiology, 14(2-3), 69-97.
Neuroscientifically Challenged. (2014, June 13). KNOW YOUR BRAIN: NUCLEUS ACCUMBENS. https://neuroscientificallychallenged.com/posts/know-your-brain-nucleus-accumbens
Nobili, A., Latagliata, E. C., Viscomi, M. T., Cavallucci, V., Cutuli, D., Giacovazzo, G., Krashia, P., Romana Rizzo, F., Marino, R., Federici, M., De Bartolo, P., Aversa, D., Concetta Dell’Acqua, M., Cordella, A., Sancandi, M., Keller, F., Petrosini, L., Puglisi-Allegra, S., Biagio Mercuri, N., Coccurello, R., Berretta, N. & D’Amelio, M. (2017). Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer’s disease. Nature communications, 8(1), 1-14.
Salgado, S., & Kaplitt, M. G. (2015). The nucleus accumbens: a comprehensive review. Stereotactic and functional neurosurgery, 93(2), 75-93.
Wise, R. A. (1982). Neuroleptics and operant behavior: the anhedonia hypothesis. Behavioral and brain sciences, 5(1), 39-53.
Zabek, M., Sobstyl, M., Koziara, H., & Dzierzecki, S. (2008). Deep brain stimulation of the right nucleus accumbens in a patient with Tourette syndrome. Case report. Neurologia i neurochirurgia polska, 42(6), 554-559.

